
Soutenir la cause
Rethinking Precision Medicine Through the Eyes of Biologists
DIAGNOW is a comprehensive platform
that empower Biologists Expertise
Designed by users for users
Unlock the Power of DIAGNOW in Your Field of Expertise
What you are facing, we have faced too !
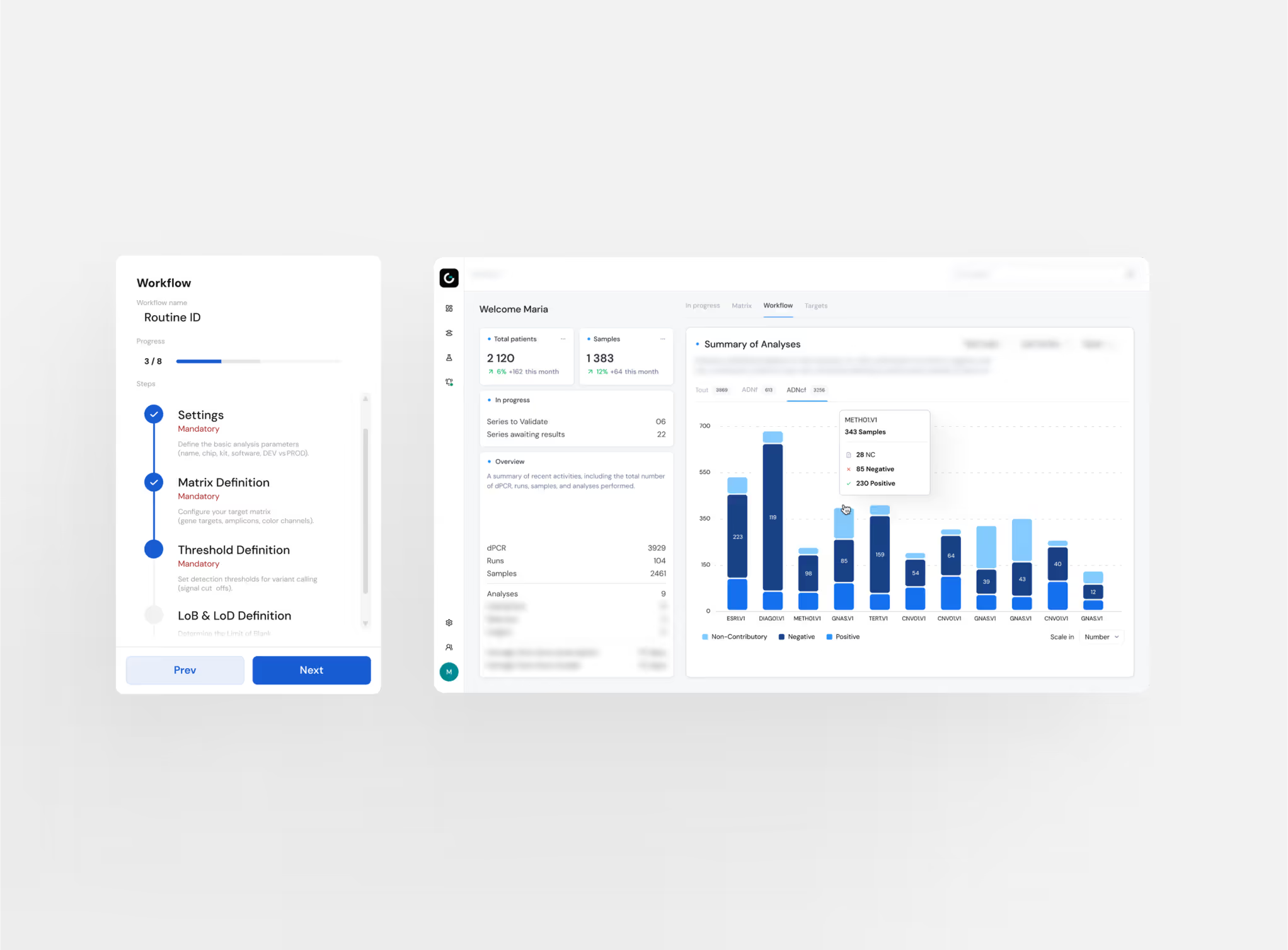
Need of customization
Understanding complex analytical workflows
The deeper the expertise, the harder becomes to share
Data fragmentation and accessibility challenges

Features That Solve Real Problems
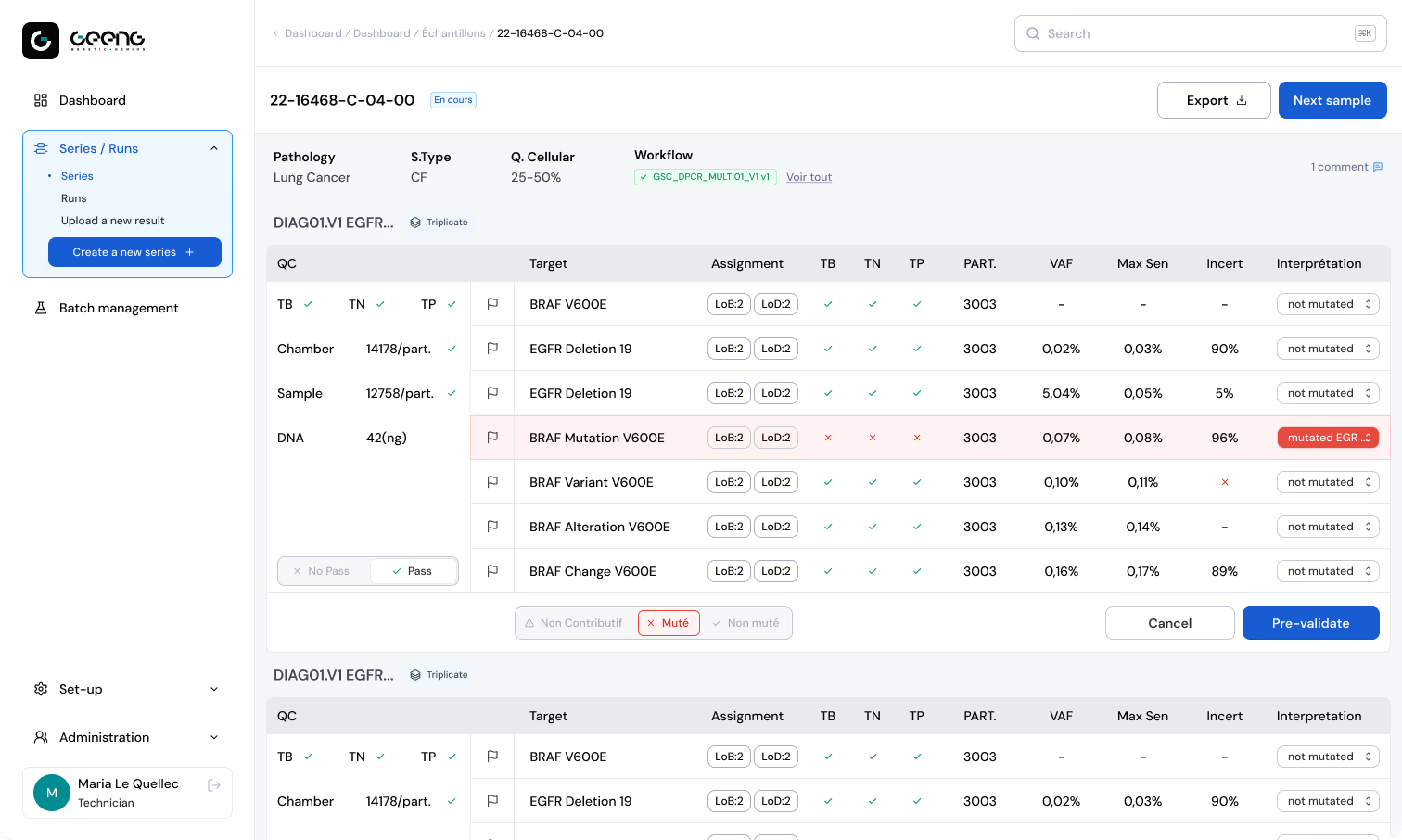
Customization and Modular Workflow Engine
Expert Data Visualization for decision-making
Automated Traceability
Knowledge Sharing & Collaboration Hub
Geeng Team
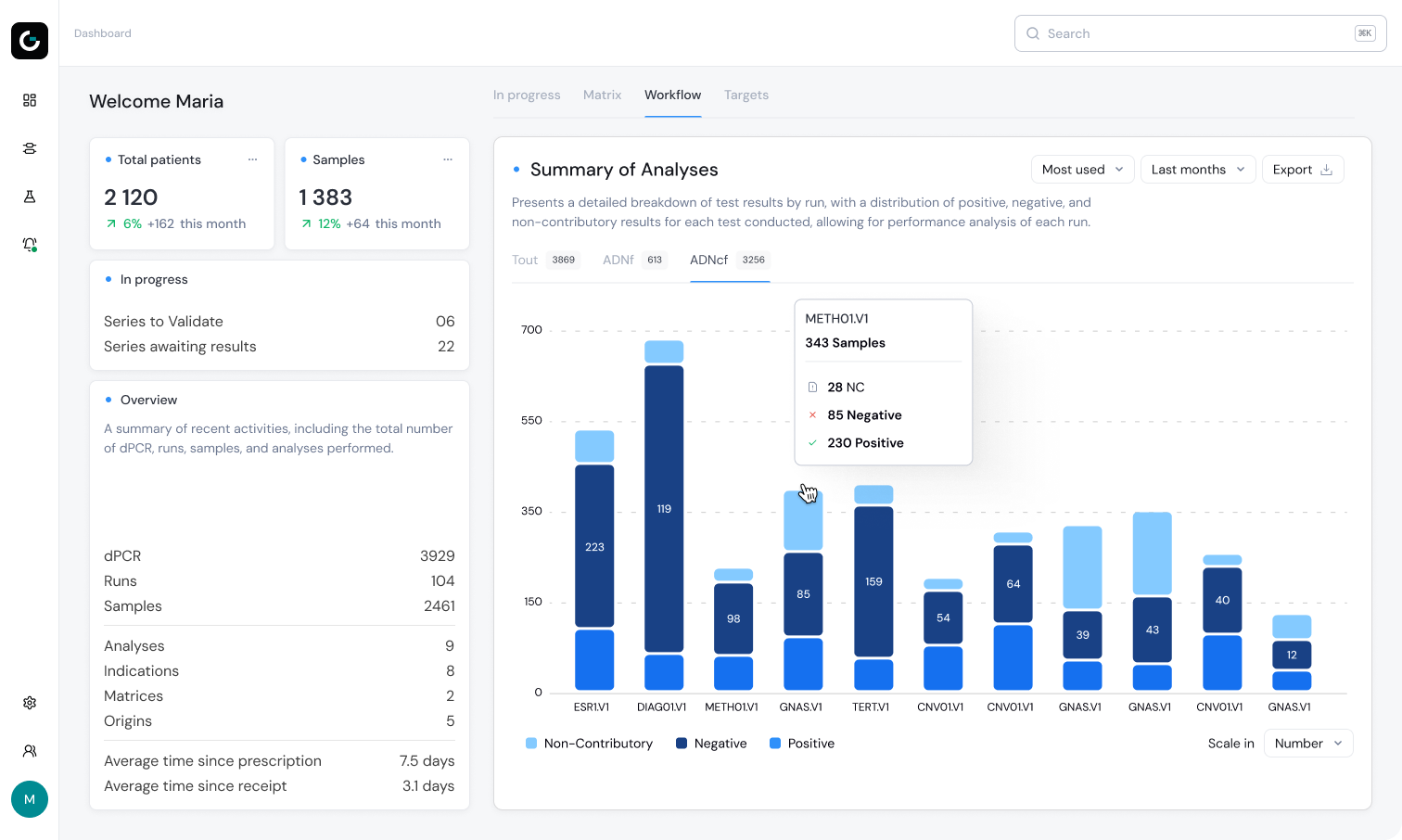
Meet the next evolution of interpreting Droplet Digital PCR Data
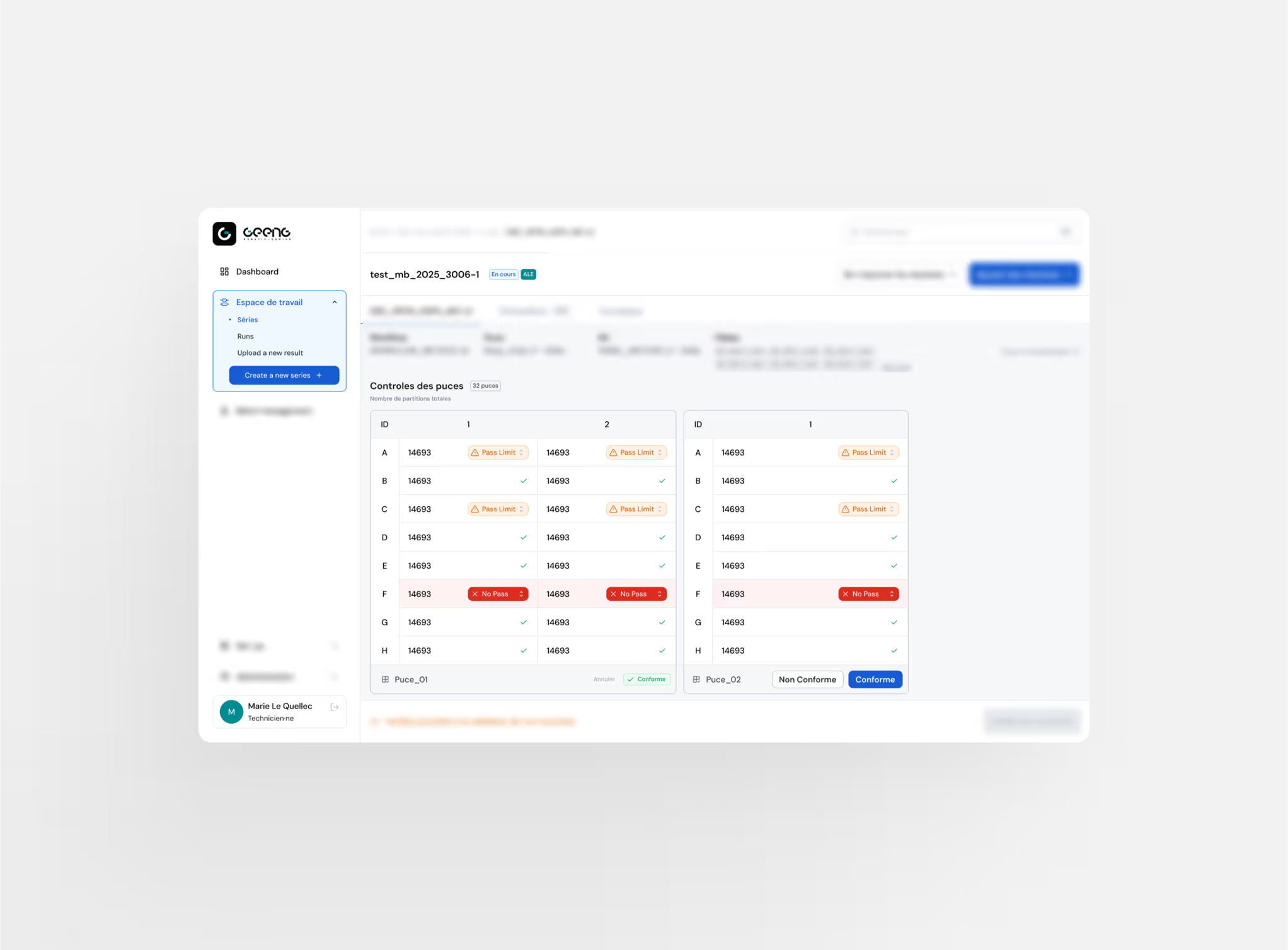
Automatic QC Controls
Continuous monitoring of positive, negative & blank controls
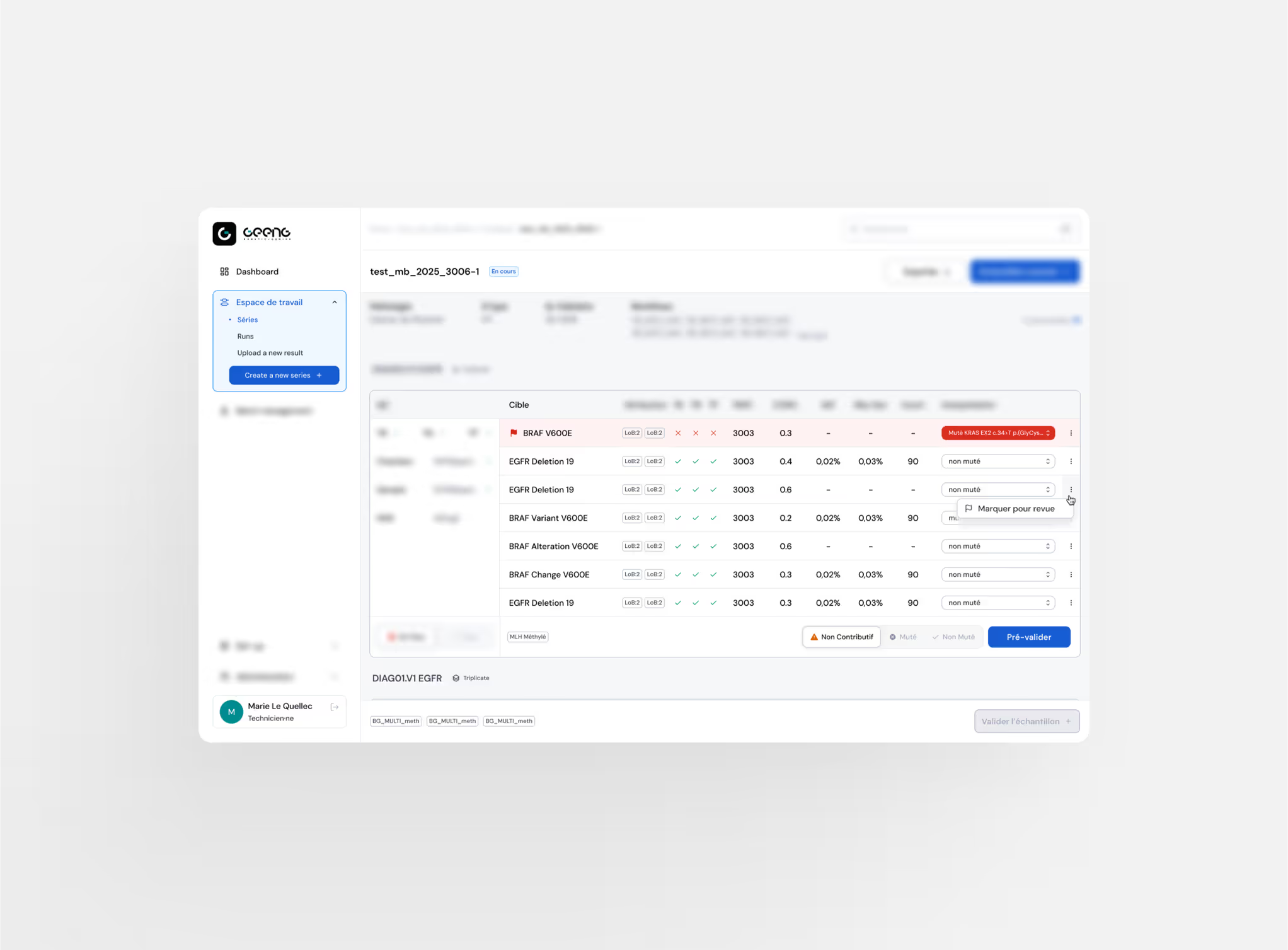
Automated Pre‑validation
Automatic pre‑validation to streamline analysis and power each steps
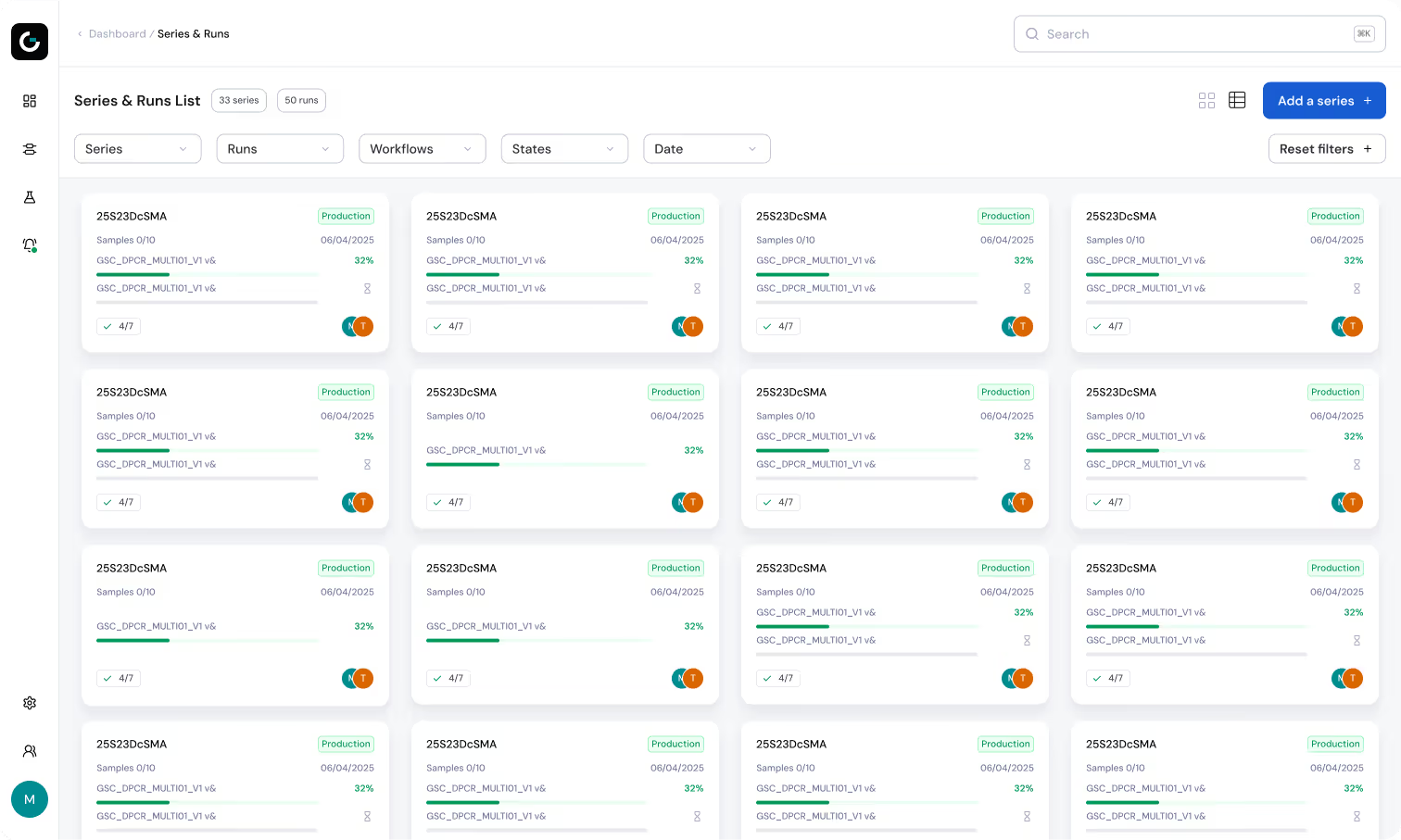
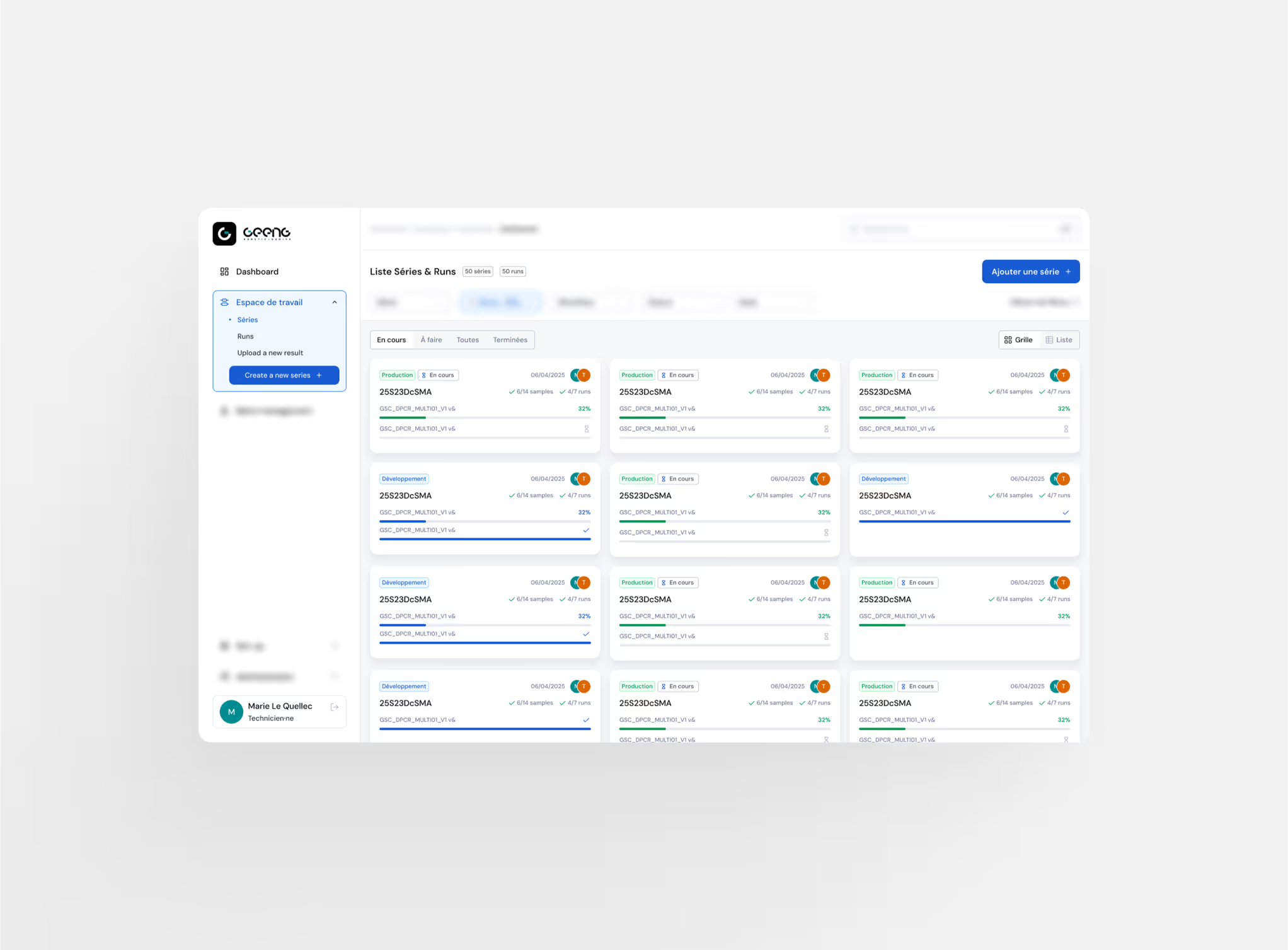
Work Management
Easily monitor your laboratory’s activity and optimize your workflows
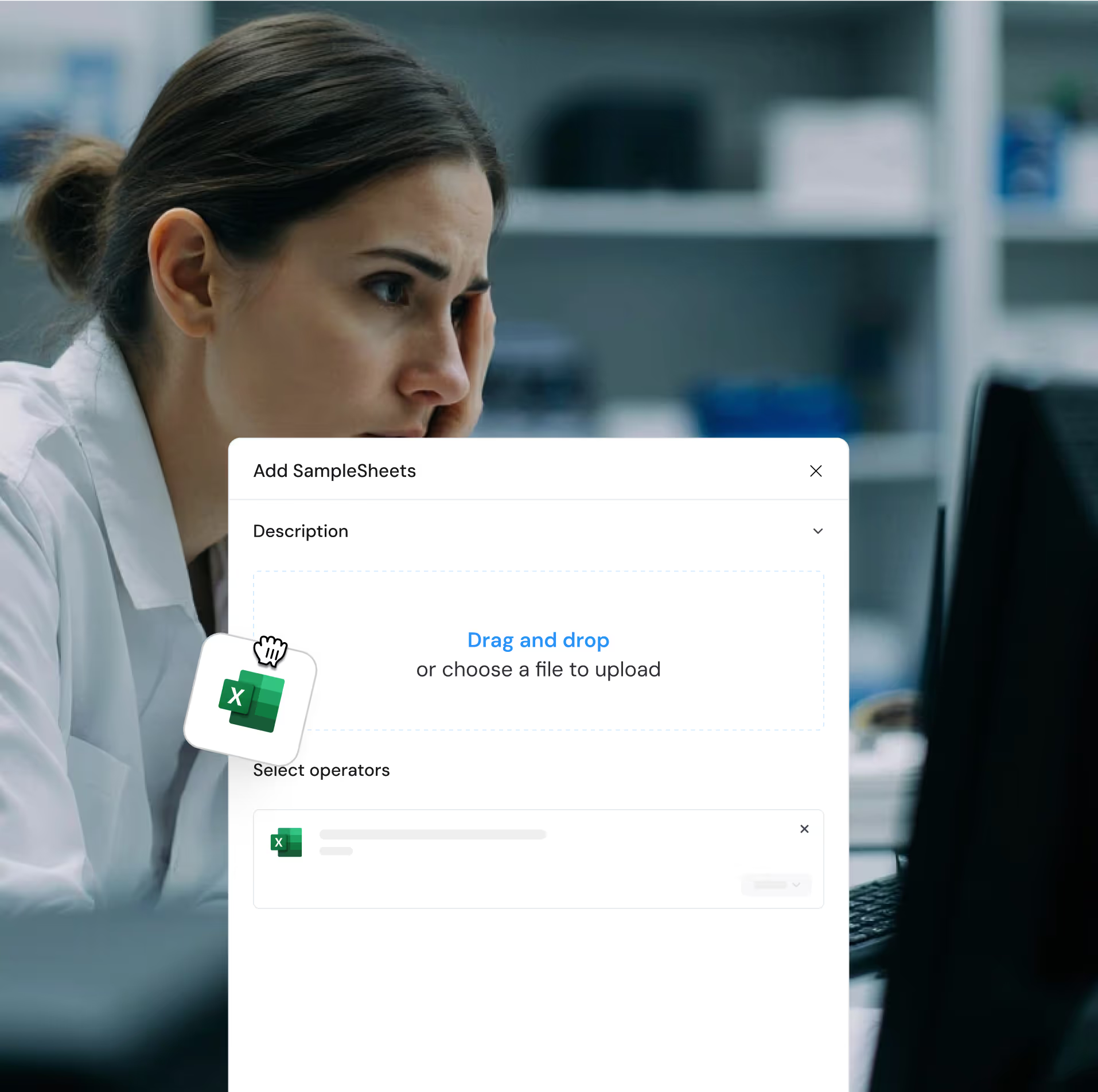
Take back control with an adaptive portal.
Design your own internal workflows with a user-centered solution. Stay ahead with the freedom to add new kits





.webp)






.webp)

All Platforms.
Fully Compatible.
Completely Trustworthy.
Intuitive Interface
Analytical Control
Security & Access
Decision Support
Sample Validation
Clinical Monitoring
Extensible Testing
Regulatory Compliance
Creative &
Tech
Geniuses
Everything you need to know before getting started.
What is DIAGNOW for biology decision making?
DIAGNOW is a cloud-based or on-premise software solution designed for analysis and interpretation of genomic and multiplex biological data (dPCR and NGS). It is dedicated to assist biologists in their daily practice. Designed to support a wide range of laboratory environments, it empowers teams in clinical diagnostics, as well as those in bioproduction and quality control, with robust data analysis and interpretation workflows providing high decision-support capabilities. For healthcare professionals it empowers diagnosis, patient monitoring (coming soon), clinical variant interpretation and therapeutic decision-making. It integrates advanced bioinformatics, AI-driven system expertise, and knowledge-based prioritization to accelerate precision medicine and biological workflows.
Does DIAGNOW support digital PCR (dPCR) data analysis and interpretation?
Yes, DIAGNOW is fully compatible with digital PCR (dPCR) workflows. It enables secure import, visualization, and interpretation of dPCR data for applications such as rare mutation detection, methylation quantification and copy number variation analysis. Results can be integrated with other omics data or reported independently for high-sensitivity clinical or QC applications.
Is DIAGNOW compatible with NGS data formats like WES, WGS, and targeted panels?
Yes, DIAGNOW will soon fully supports next-generation sequencing (NGS) data including whole-genome sequencing (WGS), whole-exome sequencing (WES), targeted gene panels. It will allow SNV/INDELs, copy number variant (CNV), structural variants (SV) analysis but also additional biomarkers such as Microsatellite instability (MSI) and tumor mutational burden (TMB)... It will accept standard file formats such as VCF and tab-delimited files for streamlined processing. For end-to-end analysis, it is built to support pipelines starting from FASTQ and BAM files.
What are the benefits of choosing a cloud-based or on-premise deployment?
Cloud deployment offers scalability, automatic updates, and global access, ideal for distributed teams. On-premise installation ensures maximum control over sensitive data, compliance with internal IT policies, and integration with secure hospital or laboratory infrastructure. However, it requires internal IT resources for deployment, maintenance, and system updates.
Can the platform integrate with LIMS or hospital EMR systems?
Yes, DIAGNOW features a robust API that allows seamless integration with Laboratory Information Management Systems (LIMS), Electronic Medical Records (EMRs), and other healthcare IT platforms to ensure automated workflows and data consistency across systems.
Is the platform compliant with HIPAA, GDPR, ISO 27001, IVDR, and FDA 21 CFR Part 11 standards for medical data?
Yes. Our platform is designed to comply with key international and European regulatory standards including HIPAA, GDPR, and FDA 21 CFR Part 11. We are actively pursuing full certification under the EU IVDR (In Vitro Diagnostic Regulation). For cloud deployments, we offer hosting on ISO 27001-certified and sovereign European cloud infrastructures that comply with EU data protection laws and France's HDS (Hébergement de Données de Santé) requirements for health data. Whether deployed in the cloud or on-premise, the platform guarantees secure data storage, encryption, access control, full audit trails, and compliance with both international and national standards for medical and health data processing.
Can clinical genomic reports be customized to fit laboratory branding and workflows?
Yes, you can fully customize reports with your lab’s logo, templates, preferred terminology, and reporting style. The system also supports multilingual output and structured formats suitable for clinical records and patient communication.
What kind of training and support is available for clinical laboratories?
We offer comprehensive onboarding, user training sessions, live technical support, and access to detailed documentation and video tutorials. Our customer success team ensures your lab is confident and effective from day one.


















